Back to Basics: Preventing Rotten Eggs (aka Reduction)
![]() This article is sponsored by Scott Laboratories. For more information on product offerings, please contact Scott Labs at 800-821-7254 or visit Scott Laboratories.
This article is sponsored by Scott Laboratories. For more information on product offerings, please contact Scott Labs at 800-821-7254 or visit Scott Laboratories.
‘This wine smells a bit like rotten eggs to me,” said grandma Margie. ‘Thanks grandma, I know I missed the boat on this wine” I replied. ‘Well, what are they teaching you at that school, I’d ask for my money back if I were you.” Got to love it, nothing like family and little tough love to put you in your place even while you think you’re on top of the world. Thankfully, for me, things have progressed a bit since then.
Rotten Eggs in wine? Well not physically, but yes, wine can end up smelling like rotten eggs when things go awry. In this article, we will explore the world of Volatile Sulfur Compounds (VSC), primarily focusing on the undesirable Sulfur-Like Off Odor (SLO) Hydrogen Sulfide (H2S) and how to prevent and correct wines with this flaw. In my opinion, behind oxidation and poor sanitation practices, this wine flaw is the most common flaw in commercial hybrid winemaking.
The words ‘Sulfur compounds” usually conjures up negative images when discussed in the context of wine. And for good reason: the subset of compounds responsible for negative aromas are SLOs with descriptors such as rotten eggs, onion, cooked cabbage, and burnt rubber to name a few.
However, it is important to recognize that not all VSCs are negative attributes in wine. In fact several VSCs are rather positive, potent aromas in wine; 3-MH (3-mercaptohexanol) and 3-MHA (3-mercaptohexylacetate) are prime examples of key VSCs that are referred to as ‘volatile thiols” and impart grapefruit and passionfruit-like aromas respectively in Sauvignon Blanc. Surely, similar VSCs are found in the cold-hardy hybrids, but to date not much volatile (aroma) profiling has been carried out on white cold-hardy hybrids (but it’s an area of active research).
Hydrogen Sulfide is the poster child for SLOs. Its formation is primarily yeast based and its odor has been described as rotten eggs or sewage like — not exactly fine wine. H2S has been measured as high as 290 mg/L (ppm), while the human detection threshold limit is somewhere in the vicinity of 1 μg/L (ppb). To put that in perspective, a one mL drop of liquid into an Olympic size swimming pool is roughly the equivalent to 1 part-per-billion (ppb).
It is important to note that there are other bad-boy SLO classes out there, such as mercaptans (rotten cabbage, onion) and disulfides (burnt rubber, garlic) that can reek havoc to a wine. If disulfides occur, they are much more difficult to correct. In general, mercaptans and disulfides are in large part due to not taking appropriate corrective action when H2S first rears its ugly head. Suffice it to say, if you can get a handle on H2S, the likelihood that you’ll have to deal with mercaptans or disulfides significantly decreases.
Causes / Formation
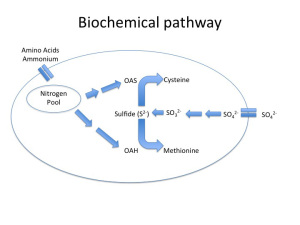
One of the best-characterized causes of H2S is due to nitrogen limitation during primary fermentation. Fig. 1a depicts a healthy yeast cell (Saccharomyces cerevisiae) undergoing the sulfate reduction pathway. What is important to note in this figure is not so much the intermediates involved but rather that there are two different elemental energy sources entering the cell, nitrogen and sulfur, that through a series of bioconversions combine to form the amino acids methionine and cysteine.
Amino acid formation is important from a cellular function standpoint because they form the building blocks essential to protein synthesis needed for yeast growth and maintenance. Without the nitrogenous precursors (OAS and OAH), there is no backbone for Sulfide to bind to, and lo and behold, H2S is secreted from the cell and a ‘reductive” odor is now present in the wine (Fig 1b). Note, reduction in this context refers to the final state of sulfur as H2S, rather than the style of winemaking that is the purposeful avoidance of oxygen (although in certain scenarios a lack of oxygen can contribute to H2S formation — see below).
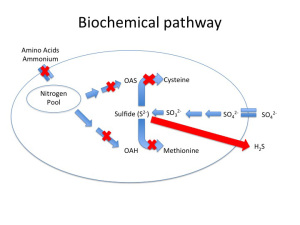
Fig 1b. H2S Production During Fermentation in Nitrogen Limited Yeast. Modified from Zoecklein Feb. 2008, WBM
There other known factors that can cause reduced SLOs in wine, several to note briefly are:
Yeast strain — There are significant differences amongst yeast strain’s capacity to produce H2S. When a strain is listed as having high nitrogen requirements, that is your cue as winemaker to take proactive measures to assure proper nutrient status, especially as it pertains to nitrogen use.
- Micronutrient deficiency — Under proficient nitrogen conditions, limiting Pantothenic Acid (a vitamin) has been linked to higher amounts of H2S production (Wang et al. 2003). This is the reason to use complex nutrient additions that contain both Nitrogen and a plethora of micronutrients.
- Prolonged exposure to gross (heavy) lees – Remove wine from gross lees as soon as possible. Gross lees defined as those lees formed 24 hours after completion of primary fermentation. It is thought that during yeast autolysis, that the breakdown of sulfur-containing material is released back into the wine. If ageing on fine (light) lees, make sure to periodically stir lees (battonage) to decrease chances of SLO formation.
- Elemental Sulfur Spray in the Vineyard — Determine if this might be a problematic source of H2S production in your wines with this nifty test. (https://www.youtube.com/watch?v=yH83vDX8ORQ)
- High Juice Turbidity — Positively correlated with mercaptans and disulfide formation. One of the major reasons to cold settle whites before fermentation.
For a more comprehensive review, see Zoecklein citation below.
Prevention / Corrective Action
The obvious answer to preventing H2S from occurring in the textbook scenario above is to provide yeast with sufficient Yeast Assimilable Nitrogen; aka YAN. Proficient YAN guidelines should be based on starting Brix (Butzke and Dukes, 1998). For instance, a starting brix of 21 requires 200 mg N/L whereas as starting brix of 25 requires 300 mg N/L. In addition to Nitrogen requirements, it should now be obvious that other nutrients are also necessary in order to avoid H2S formation. It is reasonable to assume that if a deficient amount of YAN is measured that there is a higher probability that other nutrients (micro, vitamins, minerals, etc.) are lacking.
In the hustle or bustle of harvest/crush season, a reductive note can happen to the best of us. If caught early in the fermentation process, it is best to take action as soon as possible. In primary fermentation, during the first 2/3rds of sugar depletion, a splash-racking will help ‘blow-off” formed H2S since it is highly volatile and an addition of 100 — 200 ppm DAP will prevent further formation if it is determined that Nitrogen is limiting. Meanwhile during the tail end of fermentation a splash-racking may still blow-off H2S while supplemental nitrogen will have little effect since yeast’s capacity to uptake nitrogen has drastically decreased due to the presence of alcohol. If YAN is still in check, a vitamin or mineral addition may still be of benefit.
To correct a H2S problem post-fermentation the commercial vintner has a couple of options. The classic correction for reduction (a reduction in reduction if you will) in wine is to use Copper Sulfate. Copper fining is highly effective at treating H2S because Copper Sulfate dissociates at wine pH and copper will bind with sulfide, forming a black precipitate at the bottom of your tank (Copper Sulfide). Wine can then be racked from this precipitate. Care should be taken to add an effective dose of copper but not more than is needed to correct the issue at hand because copper can also strip away positive attributes, such as the volatile thiols (mentioned above) that give a wine varietal character.
Furthermore, copper can lead to hazing issues where excess copper binds with wine proteins and can also act as a catalyst for chemical oxidation. Barring the negative side effects from using too much Copper, treating a wine with Copper often unveils positive varietal aromas that were previously masked by H2S, markedly improving the quality on the wine in hand. TTB limits the amount of copper used to treat wine at 6.0 ppm, while residual levels in wine must be at or below 0.5 ppm. If extensive amounts of copper are needed to properly treat a wine, look to yeast hulls or yeast fining techniques to reduce residual levels of copper in wine. The magic here is that yeast cell walls are rather proficient at adsorbing metals.
Another option is one of newer products on the market, Reduless from Lallemand. Reduless is a proprietary yeast-inactivated product (surprise, surprise) that is rich in copper. The advantage to using a product like Reduless over Copper Sulfate is that the addition can treat the SLO at hand, but can also round out mouthfeel characteristics. This product shines in red winemaking, where a copper sulfate treatment sometimes unveils harsh tannins in wine. Again here lab trials are warranted because too much of a good thing can also be bad.
When first encountering a SLO, it may be hard to determine if a) there is a perceivable flaw or not and b) what SLO is at hand. Before you send out your sample for quantitative chemical analysis, there are a couple of qualitative tests that are easy to do yourself. First, if H2S is suspect, simply take a glass of reduced wine and add a couple of pre-1982 pennies (95% copper) to the sample and see if it corrects your problem. If not, a more discriminatory test is the Aroma Screen Test. Gusmer sells an excellent version of this test, Sulfide Detection/Treatment Kit, with thorough literature to boot. Through a process of elimination (Fig.2), you can systematically determine what class of SLO is in hand. Note that Cadmium (Cd2+) is toxic and is used only for screening purposes and must be disposed of appropriately.
When it comes to crafting quality wine, certainly H2S is not part of the equation. Fortunately this is a wine flaw that is both easily avoided and often correctable. Hopefully this article has provided some insight on the basics of H2S formation, prevention, and corrective action. Let’s try to keep the rotten eggs out of our wines. After all, we wouldn’t want to disappoint Grandma Margie again.
Piero Spada received his MS from Cornell’s Viticulture and Enology program and was previously a winemaker in the Finger Lakes. Piero is now an independent vineyard and winery consultant based in Wisconsin and can be reached via email at pierospada.com
For more information, please see the following sources:
Linderholm, AL and LF Bisson. November/December 2005. Eliminating formation of hydrogen sulfide by Saccharomyces. PWV Journal.
Bruce Zoecklein. 2007. Sulfur-Like Off Odors in Wine: A Review of Winery Options. Virginia Tech Enology Notes.






Hi,
If I understand this correctly, you are saying the prior to fermentation, the addition of Yeast Nutrient/Energizer will help minimize the probability of this happening.
But if it is after fermentation and you are too late, then add copper to try to save the wine.
I imagine the prevention is better than being reactive.
Is there a reason not to just always use the Yeast Nutrient?
I always use Fermaid K at the typical recommended dose of a bit over a gram per gallon of must about a third of the way through ferment. Sometimes I divide the dose at 1/3 and 2/3 fermentation. I only use DAP at about mid-ferment on fruit that is overripe, damaged, underripe, etc. It is better to err on the side of usage of a complex nutrient than to err on the side of not using it.